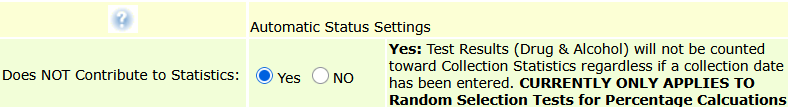
About DOT Clearinghouse Queries
The DOT Drug & Alcohol Clearinghouse requires employers (or their designated service agents) to run specific types of queries on CDL drivers for compliance. Each query type serves a different purpose depending on when the driver is being checked and what level of information is required.
DrugTestNetwork will output the data, formatted properly, into a file you can upload directly to the DOT through your Clearinghouse Account for a “batch” query. You can create a file for multiple companies or an individual company and submit them all separately as needed. Read about the Required File Format.
In batch (.txt) uploads, query types are commonly represented as numeric values:
1, 2, 3, and 4.
Query Type 1: Limited Query
Purpose: Confirms whether a driver has any drug or alcohol violations recorded in the Clearinghouse.
- What it returns: Yes/No only (no violation details)
- Consent required: General (standing) consent on file
- When to use: Annual checks for currently employed CDL drivers (when consent is already on file)
Important: If a Limited Query indicates a violation exists, you must perform a
Full Query (Type 2) to view details.
Query Type 2: Full Query
Purpose: Retrieves detailed Clearinghouse information, including violation and Return-to-Duty status.
- What it returns: Violation details, dates, RTD/SAP status (where applicable)
- Consent required: Driver electronic consent for each Full Query
- When to use: When details are required (e.g., after a violation is found or to confirm RTD status)
Full Queries are used whenever an employer needs the details behind a “violation found” result or needs to verify a driver’s eligibility status following a violation.
Query Type 3: Pre-Employment Full Query
Purpose: A mandatory Full Query performed before allowing a driver to perform safety-sensitive duties.
- What it returns: Same detailed results as a Full Query
- Consent required: Driver electronic consent
- When to use: Pre-employment screening before hiring / onboarding a CDL driver for safety-sensitive work
Key point: A Limited Query (Type 1 or Type 4) cannot be used for pre-employment purposes.
Pre-employment screening requires a Full Query.
Query Type 4: Limited Query with Automatic Consent Request
Purpose: Runs a Limited Query (Yes/No only) and, if general consent is missing, automatically requests it from the driver.
- What it returns: Yes/No only (no violation details)
- Consent behavior:
- If general consent already exists, the Limited Query proceeds normally.
- If general consent is missing, the Clearinghouse automatically sends a consent request to the driver.
- When to use: Annual Limited Queries when consent may be missing for some drivers (especially in large batch uploads)
Important: Type 4 still returns only a Limited result. If a violation is found, you must follow up with a Full Query (Type 2) to view details.
Quick “When to Use” Summary
- Type 1: Annual Limited Query when general consent is already on file.
- Type 2: Full Query when detailed results are required (including RTD/SAP status).
- Type 3: Required Full Query for pre-employment screening before safety-sensitive work.
- Type 4: Annual Limited Query when general consent may be missing; automatically requests consent if needed.
Rule of thumb:
Limited Queries (Types 1 & 4) answer: “Does a violation exist?”
Full Queries (Types 2 & 3) answer: “What are the details?”
Quick Comparison Table
| Query Type | Name | Returns Details? | Consent | Typical Use |
|---|---|---|---|---|
| 1 | Limited Query | No (Yes/No only) | General consent | Annual checks |
| 2 | Full Query | Yes | Electronic consent | Details / RTD status |
| 3 | Pre-Employment Full Query | Yes | Electronic consent | Pre-hire screening |
| 4 | Limited Query with Automatic Consent Request | No (Yes/No only) | Auto-requests general consent if missing | Annual checks at scale |